Medable
A Clinical Trials Platform
-
ClientMedable
-
Year2022 - 2023
-
RoleProduct Design Lead
-
ToolsFigma, Miro, Mentimeter
Overview
Background
The Clinical Trials Manager oversees the translation and certification of patient-centric content in the Patient App, including tasks, questions, assessments, answers, emails, SMS, and push notifications. The app assists clinical trial participants with trial-related activities and collects health data from Bluetooth-enabled wearable devices like heart rate monitors and blood pressure sensors.
Problem
The Clinical Trials Translations process is inefficient and time-consuming, as identified by questionnaires and surveys from the Customer Success Team. Each language translation takes 30-48 hours, negatively affecting productivity for both internal and external stakeholders and impacting the Institutional Review Board (IRB) preparation and submission process.
Goal
Enhance and streamline the translation process for a more efficient experience.
Core Team
Enhance and streamline the translation process for a more efficient experience.Collaborative teamwork was set up between the Product Manager, Technology Lead, and Designer/Researcher at all stages. The Product Manager, Tech Lead and myself would meet on a daily basis to circle back throughout the ideation process and communicate as a team to ensure the three of us were aligned.

Continuous Discovery Process
Outcome-driven focus
Focused on metrics-driven client-centric solutions that enhanced the Translations Process Client Experience and developing a new subscription-based product tool for clients to further improve their experience.
Conducted one-on-one Zoom shadowing sessions: Deepen understanding of users' techniques and processes while fostering enhanced relationships.
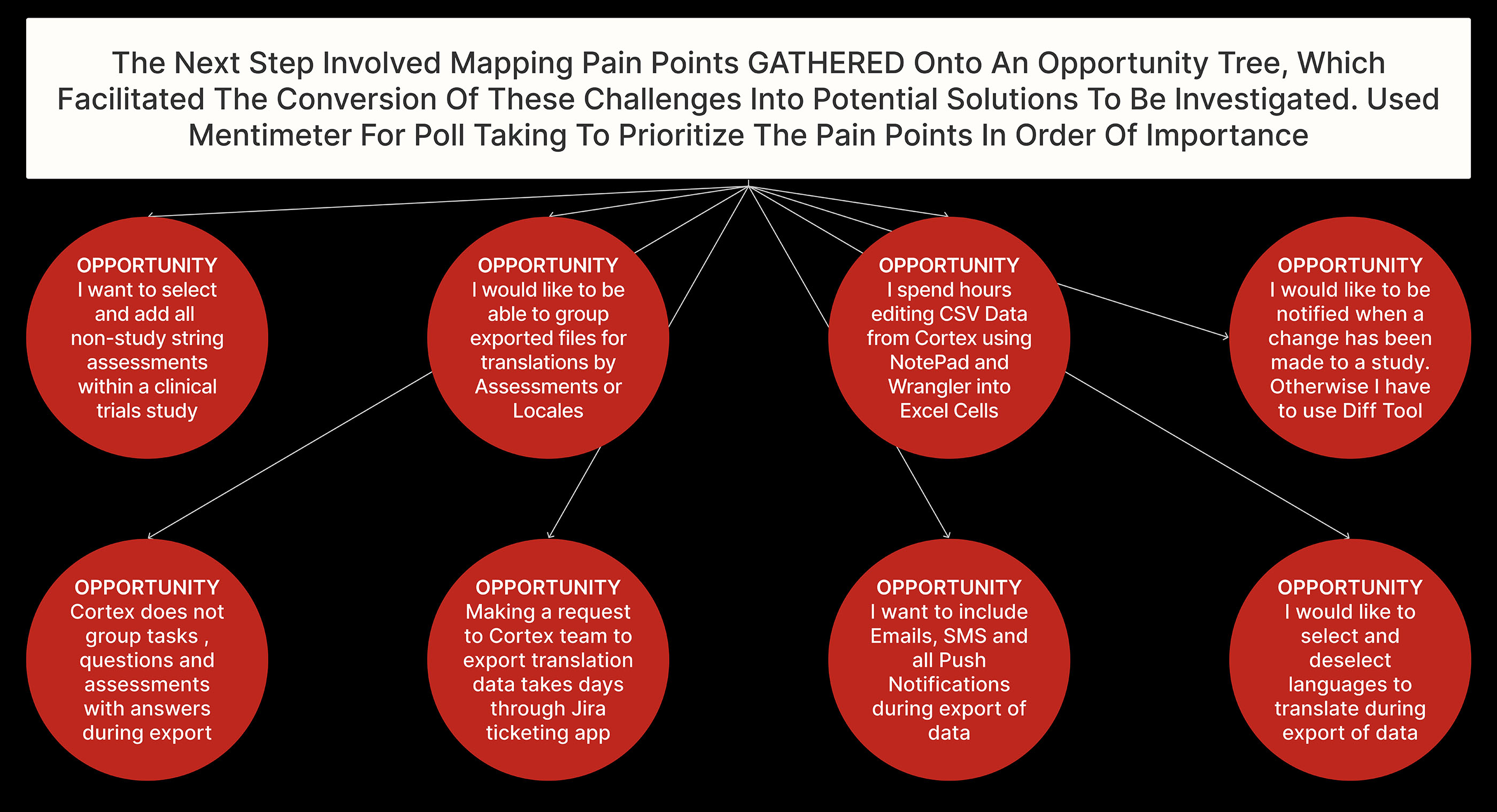
Collected user insights: Identify pain points and user perspectives through direct observation and engaging meetings, facilitating targeted improvements by conducting weekly sessions within an iterative design process.
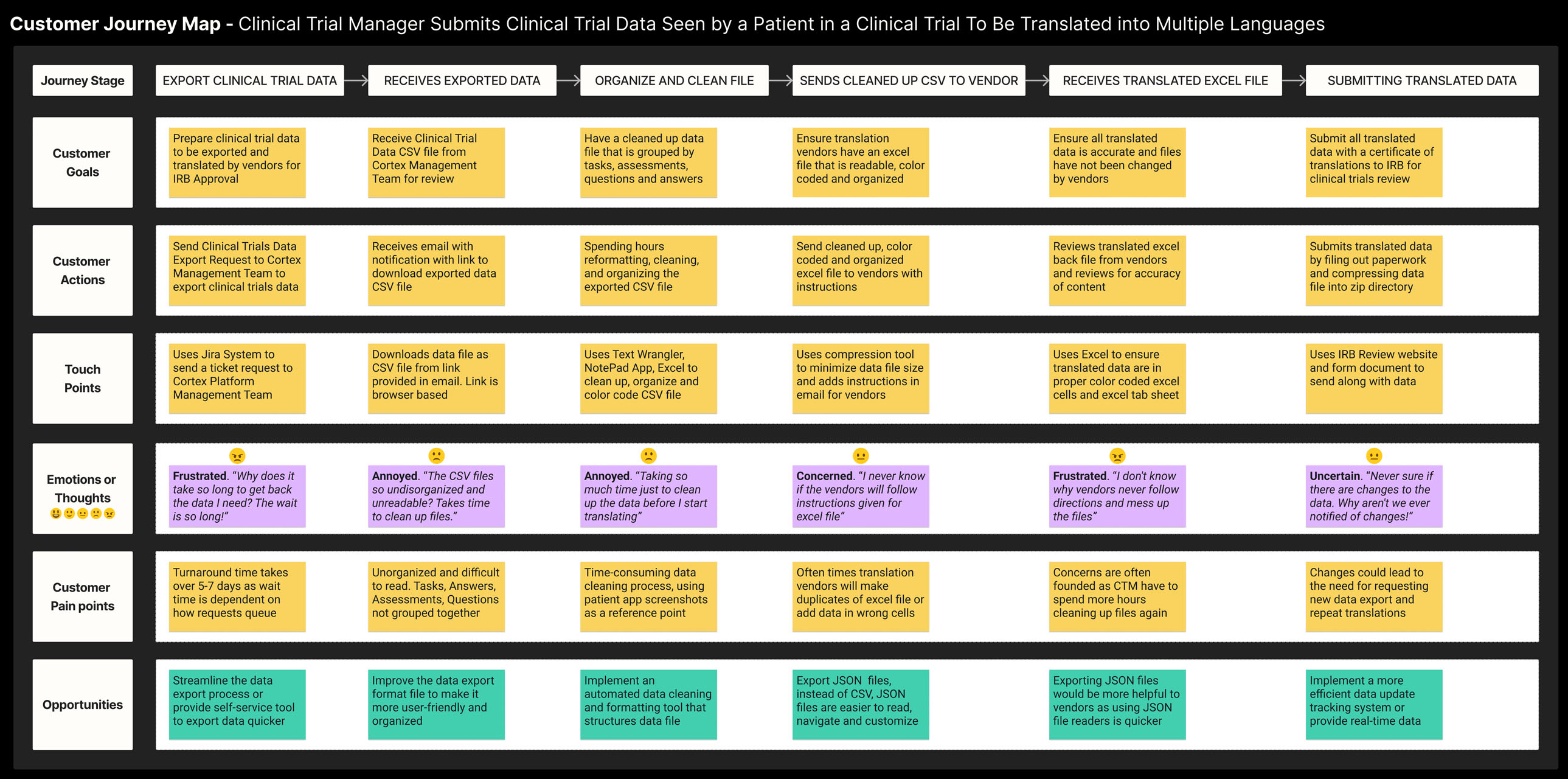
Synthesized User Research: Create Storyboard and Customer Journey Map to visualize challenges, pain points and opportunities for solutions.
Research Results
Synthesis
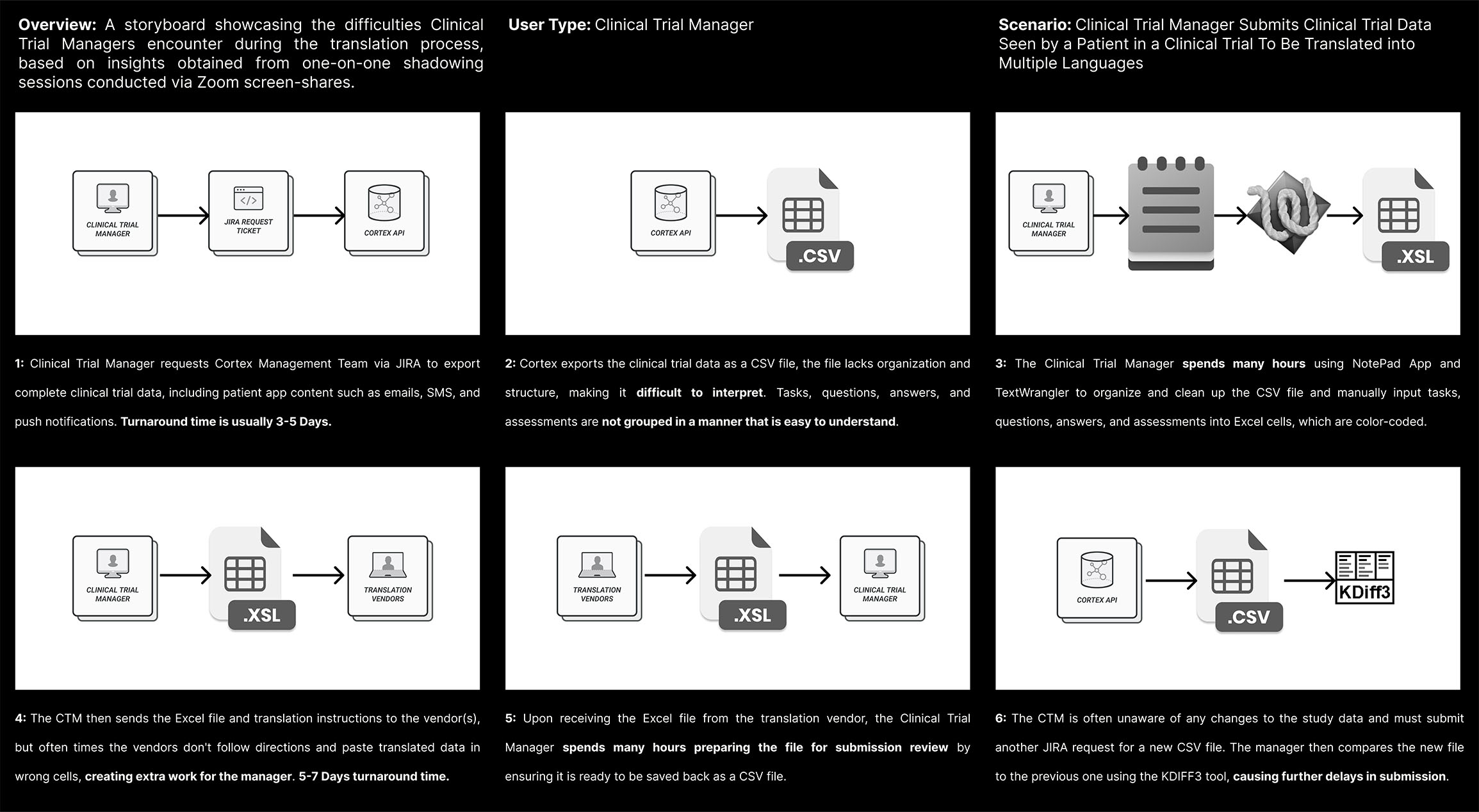
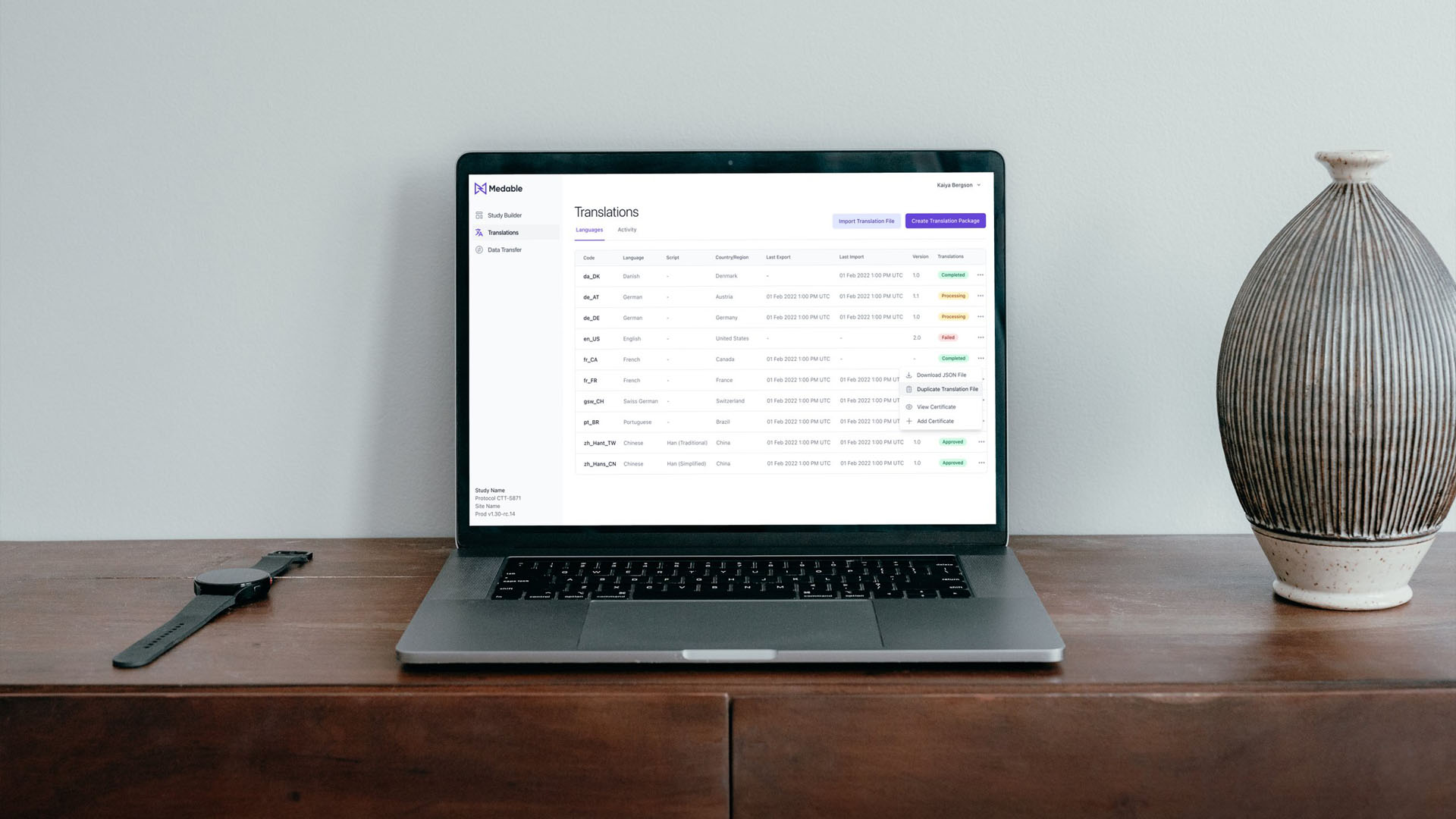
Cortex, Medable's AI Data Algorithm Platform layer, currently exports Clinical Trial Data in a CSV format. However, users have reported difficulty in interpreting the CSV content, and often need to refer to screenshots of the Patient App to make sense of it.
To streamline the translation process, users currently rely on four different tools (Excel, Word, K-Diff, and Wrangler) to convert raw data content into Excel cells. However, using these tools can be time-consuming.
Another challenge users have reported is a lack of awareness when changes are made to a study, which means they have to start the translation process all over again. This adds to the costs as translation vendors have to repeat their work.
The lengthy translation process can cause delays in the Clinical Trials Submission Review Process, which is a major concern for all stakeholders involved.
Solutions
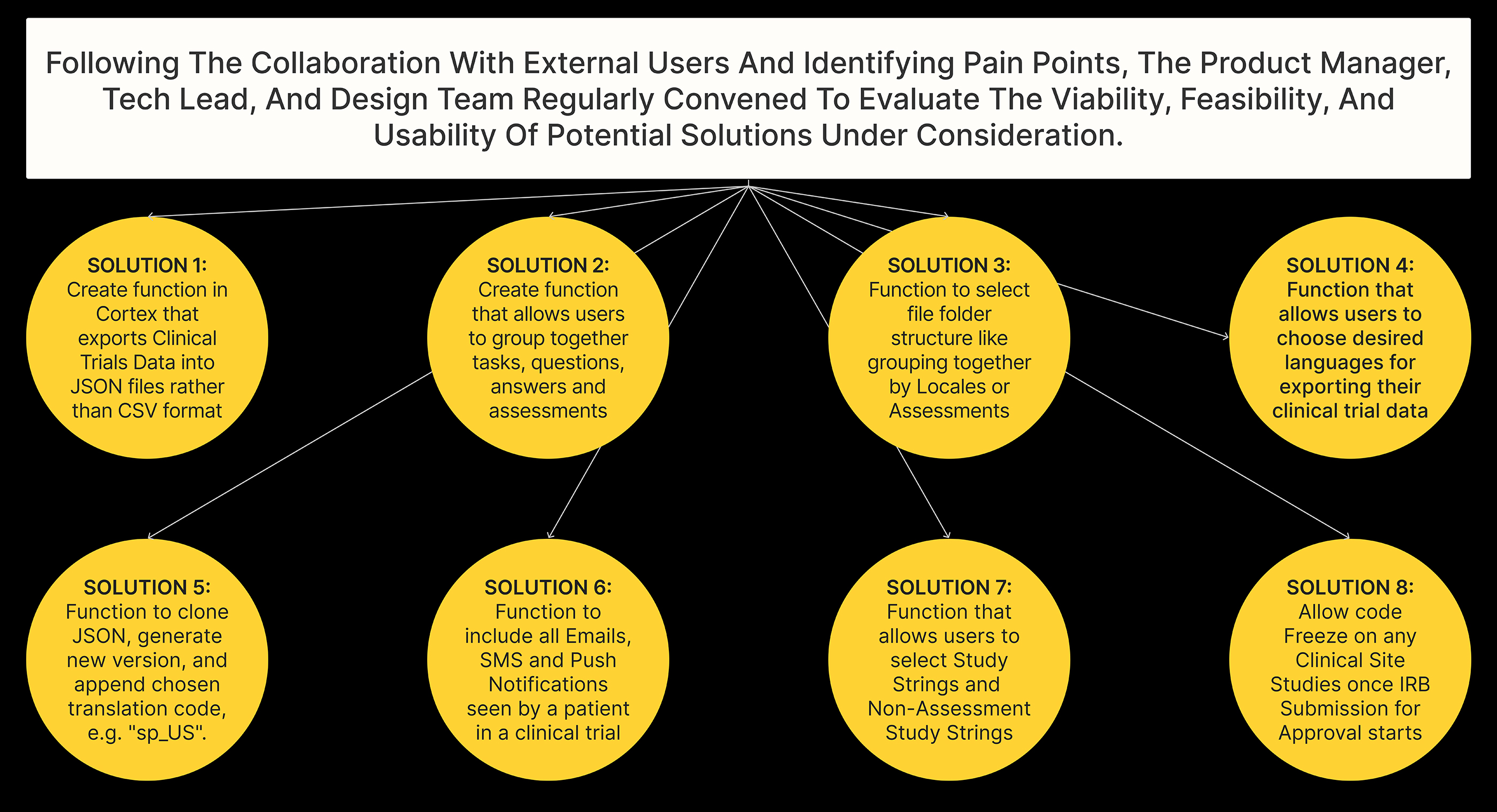
Technical
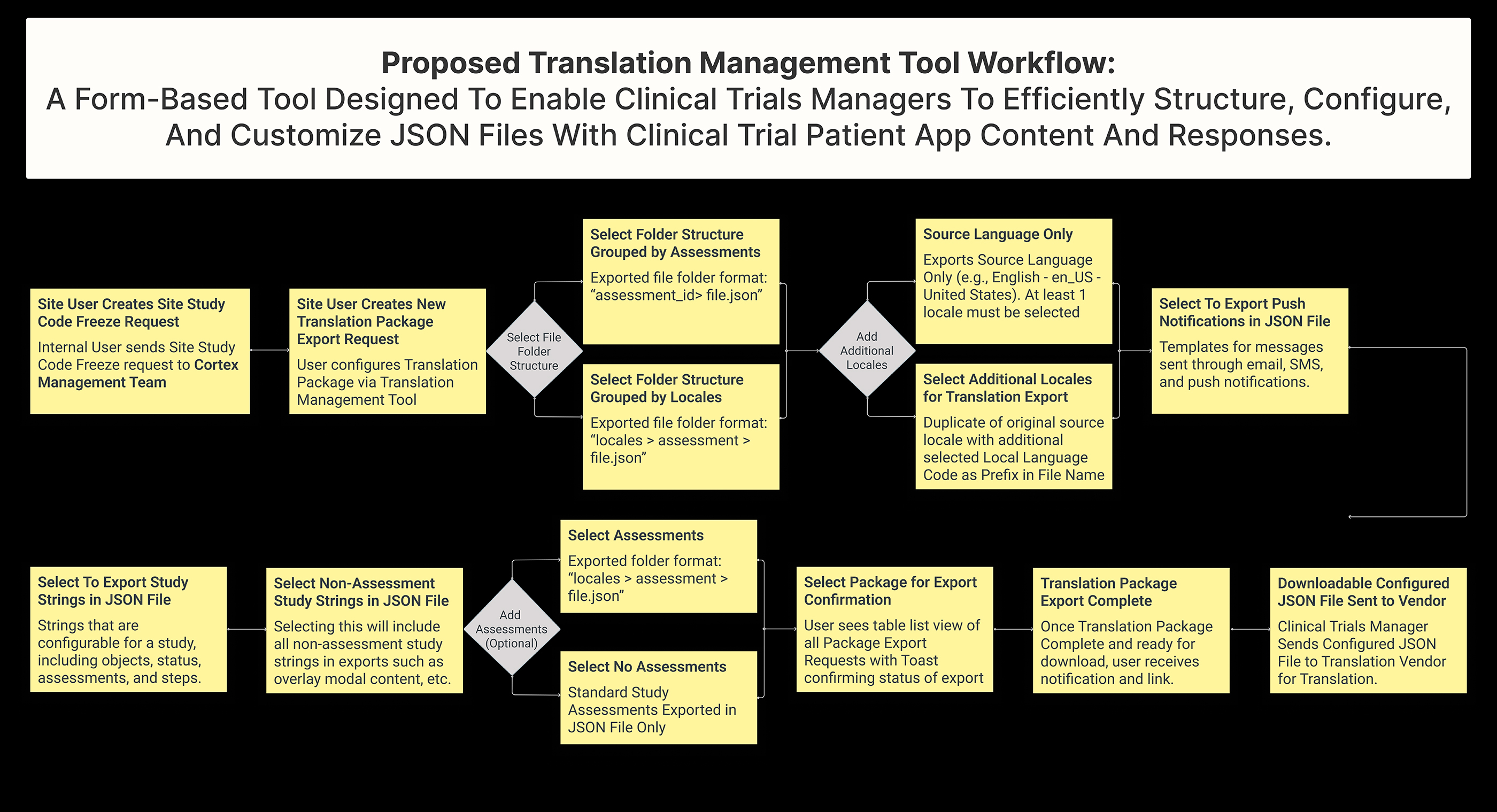
While ideating between Tech and Product, we identified a new way for Cortex, our AI Platform Layer, to export Clinical Trials Data into JSON files rather than CSV format. This will allow for more efficient grouping and organization of data content.
In the new file format, Clinical Trials Assessments, Tasks, and Answers will be grouped together in a Parent-Child format within the JSON file. This will enable users to quickly access the information they need and streamline the translation process.
Additionally, if selected, push notifications such as Emails and SMS can also be exported in the exported JSON file.
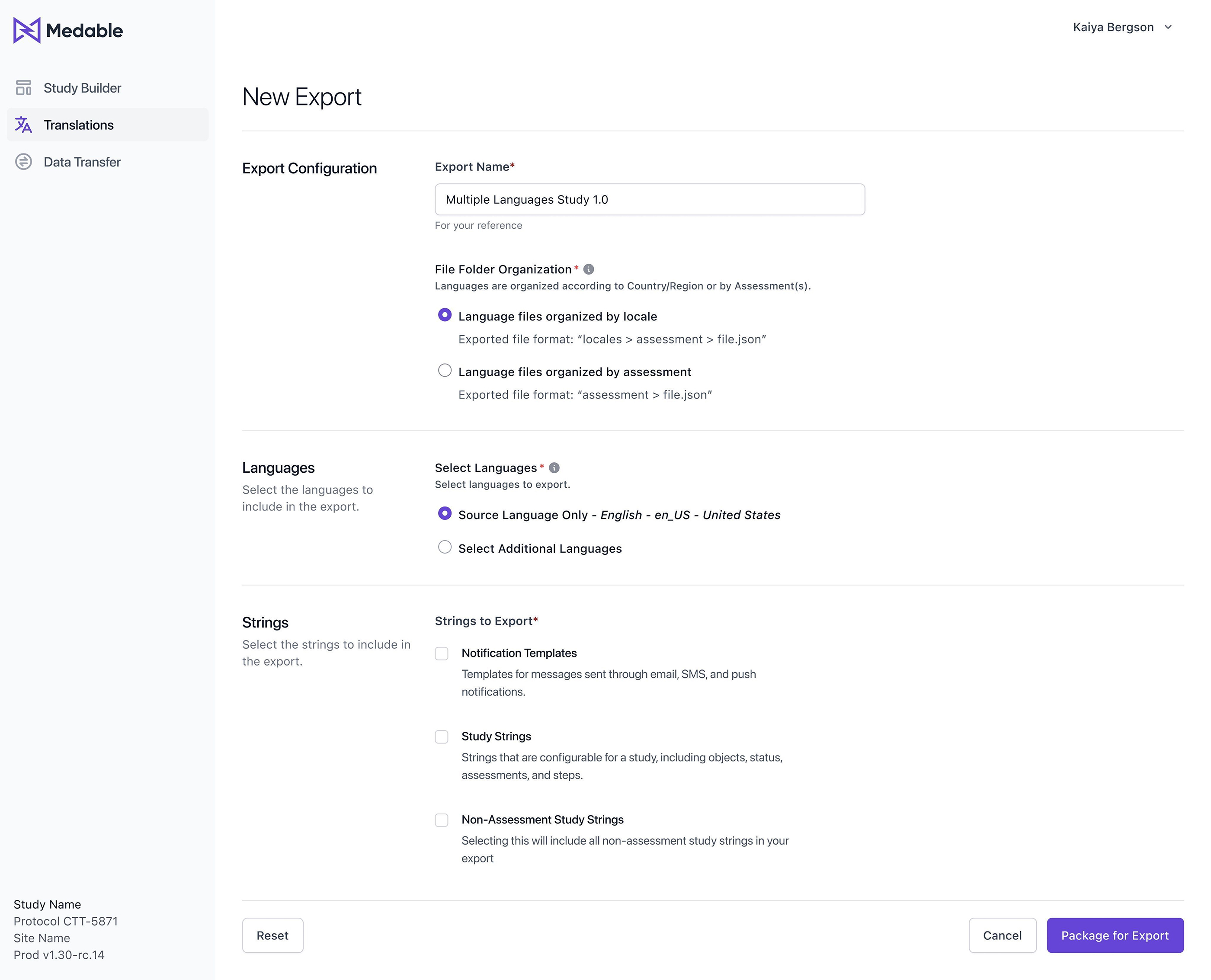
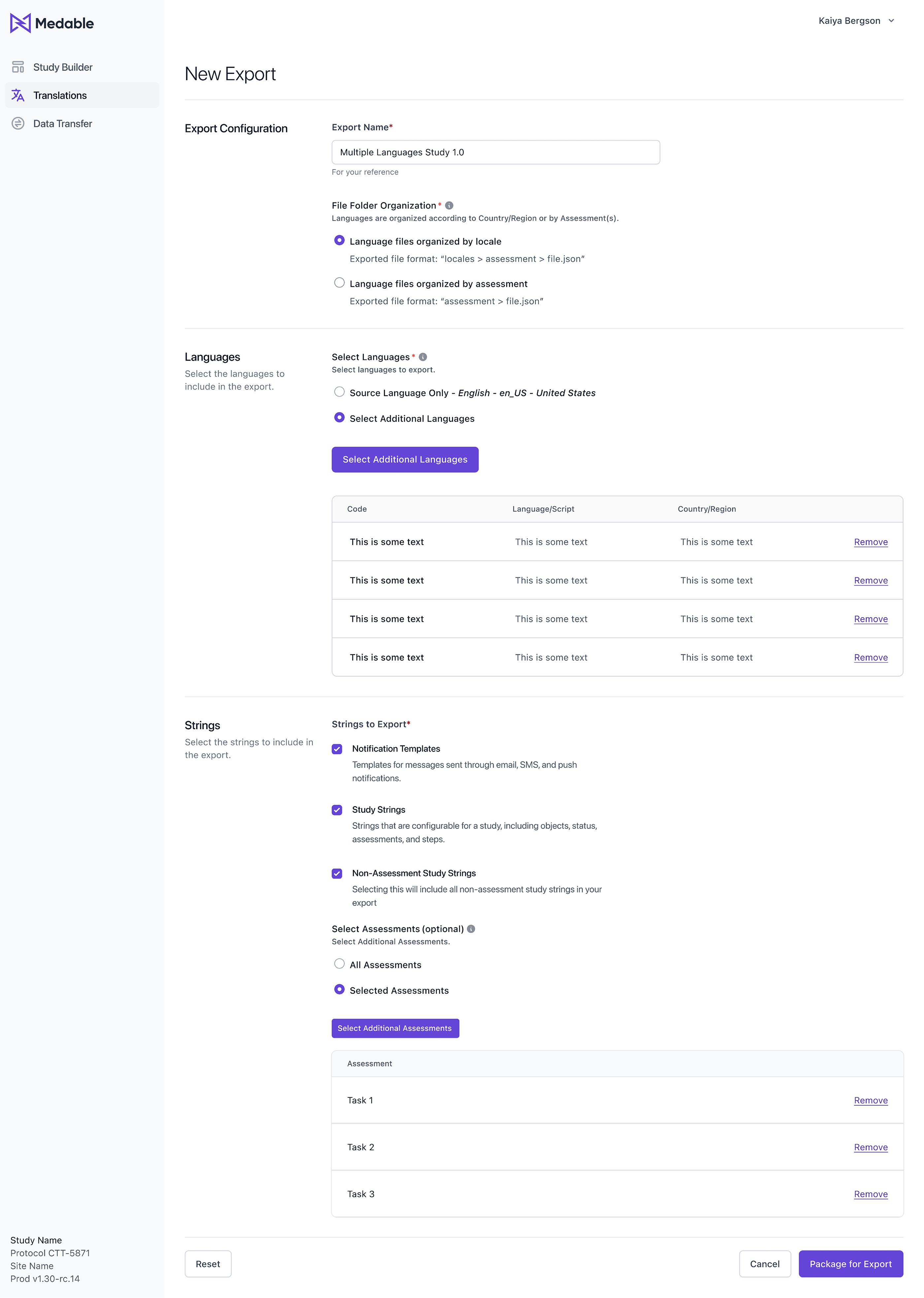
To make the translation export process even more user-friendly, we proposed an interface or tool that will allow users to configure and customize the folder directory structure and JSON file organization according to their needs.
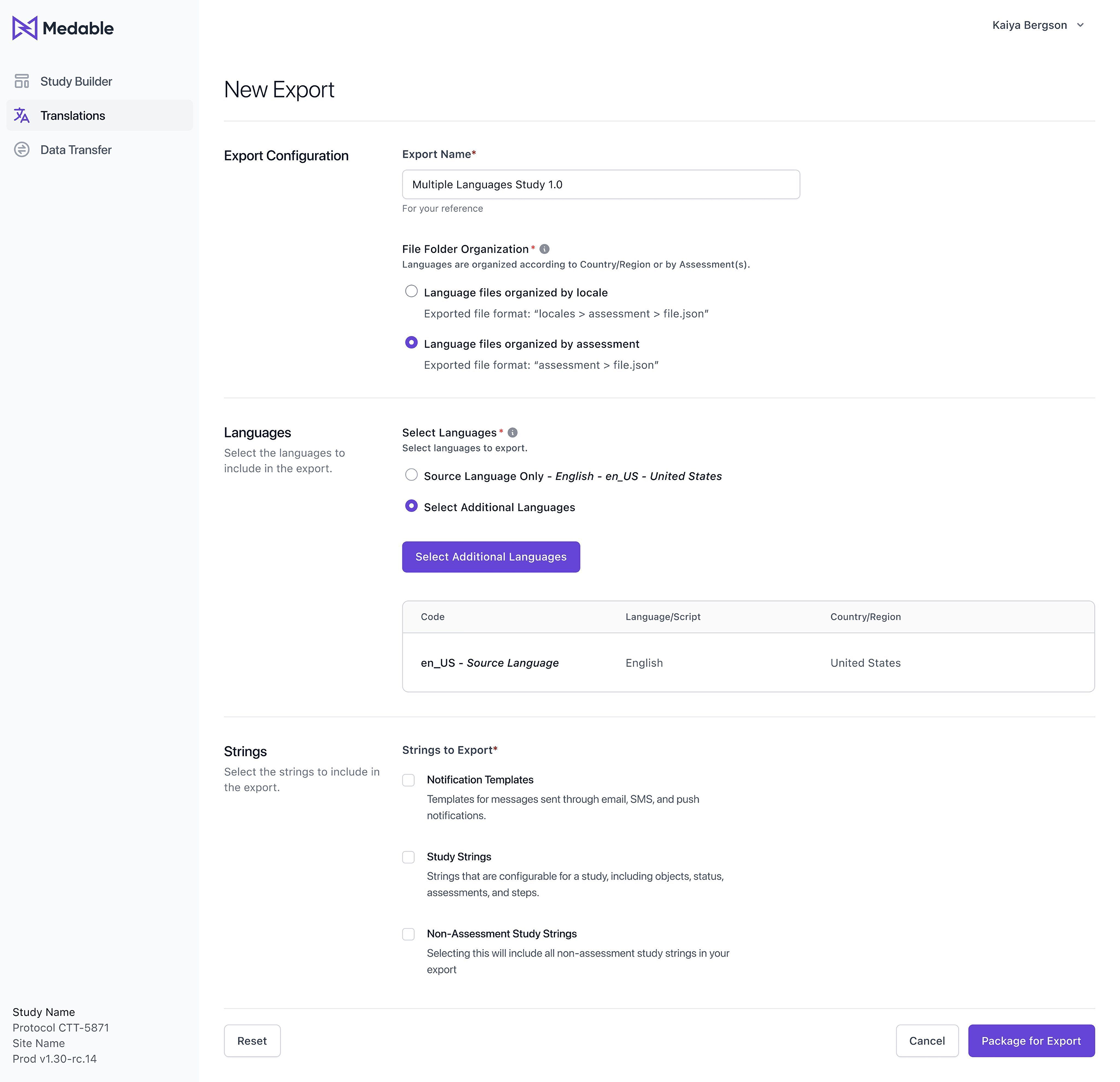
Finally, users can choose to group data by Locales (language) or by Assessments, depending on their preferences and requirements. This will give them even greater flexibility and control over the organization of their data.
Solutions
Usability & Design
Firstly, the output file will be a highly configured and structured JSON file, providing users with a clear and easily navigable format.
Users will also have the option to group data by Locale or Assessments, depending on their needs and preferences. This will allow for more efficient access to the required data and simplify the translation process.
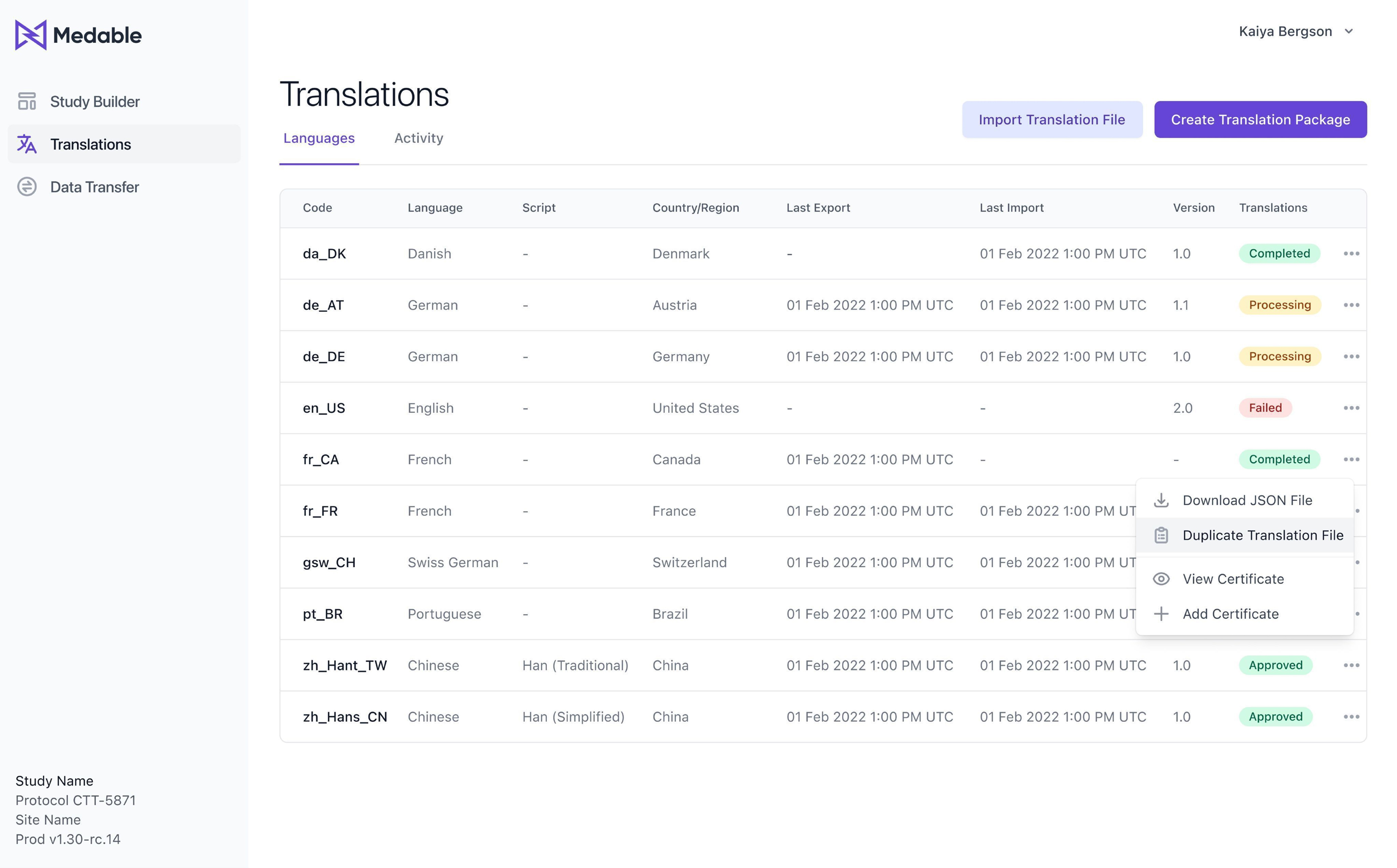
Adding a new feature which enables users to select additional languages, creating duplicate versions of the original Source Locale Version with prefixed language and script (e.g., en_us). This simplifies instructions for translation vendors and streamlines the multilingual translation process.
Finally, we added the ability to include additional Assessments in the export function, which will give users greater flexibility and control over their data.